Search
- Page Path
- HOME > Search
- Miscellaneous
- DN200434 Inhibits Vascular Smooth Muscle Cell Proliferation and Prevents Neointima Formation in Mice after Carotid Artery Ligation
- Sudeep Kumar, Jonghwa Jin, Hyeon Young Park, Mi-Jin Kim, Jungwook Chin, Sungwoo Lee, Jina Kim, Jung-Guk Kim, Yeon-Kyung Choi, Keun-Gyu Park
- Endocrinol Metab. 2022;37(5):800-809. Published online September 28, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1462
- 3,063 View
- 201 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
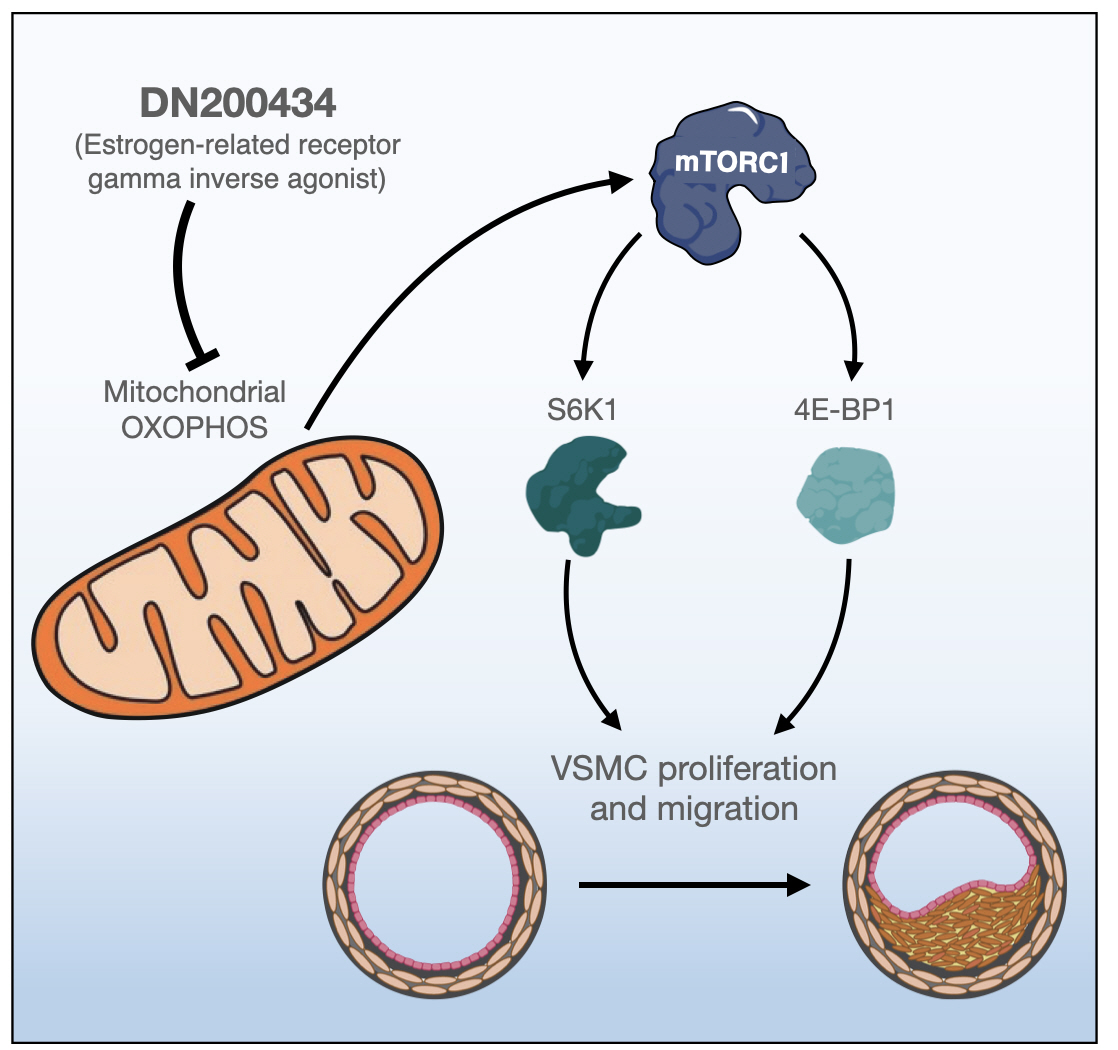
Excessive proliferation and migration of vascular smooth muscle cells (VSMCs), which contributes to the development of occlusive vascular diseases, requires elevated mitochondrial oxidative phosphorylation to meet the increased requirements for energy and anabolic precursors. Therefore, therapeutic strategies based on blockade of mitochondrial oxidative phosphorylation are considered promising for treatment of occlusive vascular diseases. Here, we investigated whether DN200434, an orally available estrogen receptor-related gamma inverse agonist, inhibits proliferation and migration of VSMCs and neointima formation by suppressing mitochondrial oxidative phosphorylation.
Methods
VSMCs were isolated from the thoracic aortas of 4-week-old Sprague-Dawley rats. Oxidative phosphorylation and the cell cycle were analyzed in fetal bovine serum (FBS)- or platelet-derived growth factor (PDGF)-stimulated VSMCs using a Seahorse XF-24 analyzer and flow cytometry, respectively. A model of neointimal hyperplasia was generated by ligating the left common carotid artery in male C57BL/6J mice.
Results
DN200434 inhibited mitochondrial respiration and mammalian target of rapamycin complex 1 activity and consequently suppressed FBS- or PDGF-stimulated proliferation and migration of VSMCs and cell cycle progression. Furthermore, DN200434 reduced carotid artery ligation-induced neointima formation in mice.
Conclusion
Our data suggest that DN200434 is a therapeutic option to prevent the progression of atherosclerosis. -
Citations
Citations to this article as recorded by- Jatrorrhizine inhibits Piezo1 activation and reduces vascular inflammation in endothelial cells
Tianying Hong, Xianmei Pan, Han Xu, Zhijuan Zheng, Lizhen Wen, Jing Li, Mingfeng Xia
Biomedicine & Pharmacotherapy.2023; 163: 114755. CrossRef
- Jatrorrhizine inhibits Piezo1 activation and reduces vascular inflammation in endothelial cells

- Diabetes, Obesity and Metabolism
- Year-Long Trend in Glycated Hemoglobin Levels in Patients with Type 2 Diabetes during the COVID-19 Pandemic
- Jonghwa Jin, Seong Wook Lee, Won-Ki Lee, Jae-Han Jeon, Jung-Guk Kim, In-Kyu Lee, Yeon-Kyung Choi, Keun-Gyu Park
- Endocrinol Metab. 2021;36(5):1142-1146. Published online October 21, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1154
- 3,903 View
- 148 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
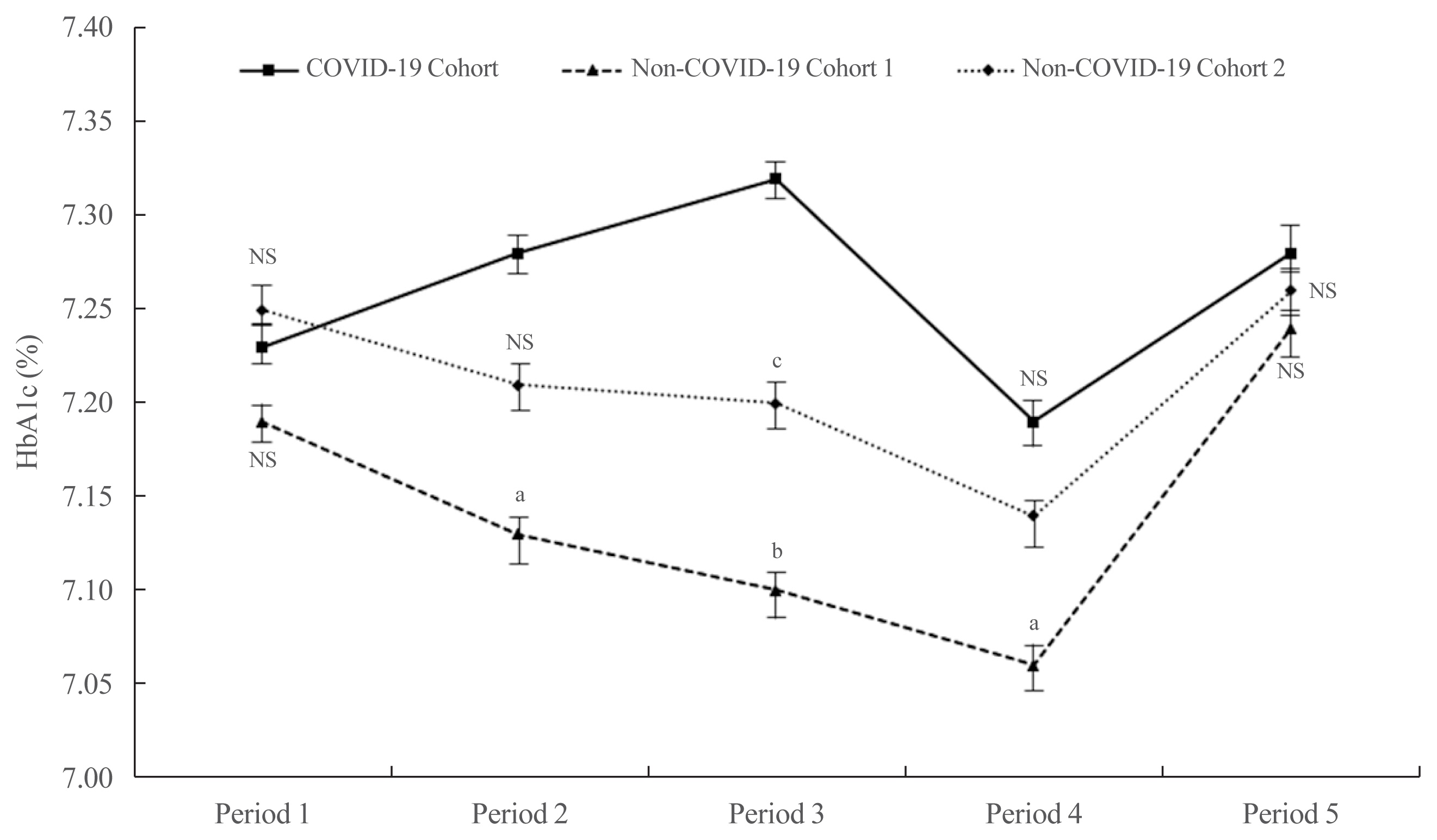
ePub - It has been suggested that the coronavirus disease 2019 (COVID-19) pandemic has had a negative impact on glycemic control in patients with type 2 diabetes mellitus (T2DM). However, no study has examined yearly trends in glycated hemoglobin (HbA1c) levels after the start of the COVID-19 outbreak. Here, we performed a retrospective analysis of HbA1c concentrations during the early period of the COVID-19 outbreak (COVID-19 cohort) and then compared the yearly trend in the mean HbA1c level, along with fluctuations in HbA1c levels, with those during previous years (non-COVID-19 cohorts). We observed that the mean HbA1c level in patients with T2DM increased during the first 6 months of the COVID-19 outbreak. After 6 months, HbA1c levels in the COVID-19 cohort returned to levels seen in the non-COVID-19 cohorts. The data suggest that vulnerable patients with T2DM should be monitored closely during the early period of a pandemic to ensure they receive appropriate care.
-
Citations
Citations to this article as recorded by- Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey
Abdulbari Bener, Murat Atmaca, Abdulla O. A. A. Al-Hamaq, Antonio Ventriglio
Brain Sciences.2024; 14(4): 377. CrossRef - A Hybrid Model of In-Person and Telemedicine Diabetes Education and Care for Management of Patients with Uncontrolled Type 2 Diabetes Mellitus: Findings and Implications from a Multicenter Prospective Study
Ayla M. Tourkmani, Turki J. Alharbi, Abdulaziz M. Bin Rsheed, Azzam F. Alotaibi, Mohammed S. Aleissa, Sultan Alotaibi, Amal S. Almutairi, Jancy Thomson, Ahlam S. Alshahrani, Hadil S. Alroyli, Hend M. Almutairi, Mashael A. Aladwani, Eman R. Alsheheri, Hyfa
Telemedicine Reports.2024; 5(1): 46. CrossRef - The indirect impact of the COVID-19 pandemic on people with type 2 diabetes mellitus and without COVID-19 infection: Systematic review and meta-analysis
Zhuoran Hu, Hin Moi Youn, Jianchao Quan, Lily Luk Siu Lee, Ivy Lynn Mak, Esther Yee Tak Yu, David Vai-Kiong Chao, Welchie Wai Kit Ko, Ian Chi Kei Wong, Gary Kui Kai Lau, Chak Sing Lau, Cindy Lo Kuen Lam, Eric Yuk Fai Wan
Primary Care Diabetes.2023; 17(3): 229. CrossRef - Evaluating Effects of Virtual Diabetes Group Visits in Community Health Centers During the COVID-19 Pandemic
Tracy Dinh, Erin M Staab, Daisy Nuñez, Mengqi Zhu, Wen Wan, Cynthia T Schaefer, Amanda Campbell, Michael Quinn, Arshiya A Baig
Journal of Patient Experience.2023;[Epub] CrossRef - Cardiovascular-related health behavior changes: lessons from the COVID-19 pandemic and post-pandemic challenges
Inha Jung, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2023; 5(4): 99. CrossRef
- Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey

- Lobeglitazone, a Novel Peroxisome Proliferator-Activated Receptor γ Agonist, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
- Kwi-Hyun Bae, Jung Beom Seo, Yun-A Jung, Hye-Young Seo, Sun Hee Kang, Hui-Jeon Jeon, Jae Man Lee, Sungwoo Lee, Jung-Guk Kim, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
- Endocrinol Metab. 2017;32(1):115-123. Published online February 28, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.115
- 4,856 View
- 77 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Renal tubulointerstitial fibrosis is a common feature of the final stage of nearly all cause types of chronic kidney disease. Although classic peroxisome proliferator-activated receptor γ (PPARγ) agonists have a protective effect on diabetic nephropathy, much less is known about their direct effects in renal fibrosis. This study aimed to investigate possible beneficial effects of lobeglitazone, a novel PPARγ agonist, on renal fibrosis in mice.
Methods We examined the effects of lobeglitazone on renal tubulointerstitial fibrosis in unilateral ureteral obstruction (UUO) induced renal fibrosis mice. We further defined the role of lobeglitazone on transforming growth factor (TGF)-signaling pathways in renal tubulointerstitial fibrosis through
in vivo andin vitro study.Results Through hematoxylin/eosin and sirius red staining, we observed that lobeglitazone effectively attenuates UUO-induced renal atrophy and fibrosis. Immunohistochemical analysis in conjunction with quantitative reverse transcription polymerase chain reaction and Western blot analysis revealed that lobeglitazone treatment inhibited UUO-induced upregulation of renal Smad-3 phosphorylation, α-smooth muscle actin, plasminogen activator inhibitor 1, and type 1 collagen.
In vitro experiments with rat mesangial cells and NRK-49F renal fibroblast cells suggested that the effects of lobeglitazone on UUO-induced renal fibrosis are mediated by inhibition of the TGF-β/Smad signaling pathway.Conclusion The present study demonstrates that lobeglitazone has a protective effect on UUO-induced renal fibrosis, suggesting that its clinical applications could extend to the treatment of non-diabetic origin renal disease.
-
Citations
Citations to this article as recorded by- The modulation effects of plant‐derived bioactive ingredients on chronic kidney disease: Focus on the gut–kidney axis
Shiyan Jian, Kang Yang, Lingna Zhang, Limeng Zhang, Zhongquan Xin, Chaoyu Wen, Shansong He, Jinping Deng, Baichuan Deng
Food Frontiers.2023; 4(1): 262. CrossRef - Druggability of lipid metabolism modulation against renal fibrosis
Yuan-yuan Chen, Xiao-guang Chen, Sen Zhang
Acta Pharmacologica Sinica.2022; 43(3): 505. CrossRef - Lobeglitazone attenuates fibrosis in corneal fibroblasts by interrupting TGF-beta-mediated Smad signaling
Selikem Nuwormegbe, Na-Young Park, Sun Woong Kim
Graefe's Archive for Clinical and Experimental Ophthalmology.2022; 260(1): 149. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes & Metabolism Journal.2021; 45(3): 326. CrossRef - Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
Jun-Qing Jin, Jeong-Sun Han, Jeonghoon Ha, Han-Sang Baek, Dong-Jun Lim
Endocrinology and Metabolism.2021; 36(5): 1095. CrossRef - Protocol for a preclinical systematic review and meta-analysis of pharmacological targeting of peroxisome proliferator-activated receptors in experimental renal injury
William P Martin, Yeong H D Chuah, Emer Conroy, Alison L Reynolds, Conor Judge, Francisco J López-Hernández, Carel W le Roux, Neil G Docherty
BMJ Open Science.2021;[Epub] CrossRef - Stevioside inhibits unilateral ureteral obstruction‐induced kidney fibrosis and upregulates renal PPARγ expression in mice
Wei Shen, Ke Fan, Ying Zhao, Junyan Zhang, Meilin Xie
Journal of Food Biochemistry.2020;[Epub] CrossRef - FBW7 Regulates the Autophagy Signal in Mesangial Cells Induced by High Glucose
Chenlin Gao, Fang Fan, Jiao Chen, Yang Long, Shi Tang, Chunxia Jiang, Yong Xu
BioMed Research International.2019; 2019: 1. CrossRef - Treatment with Lobeglitazone Attenuates Hepatic Steatosis in Diet-Induced Obese Mice
Sorim Choung, Kyong Hye Joung, Bo Ram You, Sang Ki Park, Hyun Jin Kim, Bon Jeong Ku
PPAR Research.2018; 2018: 1. CrossRef - VCE‐004.3, a cannabidiol aminoquinone derivative, prevents bleomycin‐induced skin fibrosis and inflammation through PPARγ‐ and CB2 receptor‐dependent pathways
Carmen del Rio, Irene Cantarero, Belén Palomares, María Gómez‐Cañas, Javier Fernández‐Ruiz, Carolina Pavicic, Adela García‐Martín, Maria Luz Bellido, Rafaela Ortega‐Castro, Carlos Pérez‐Sánchez, Chary López‐Pedrera, Giovanni Appendino, Marco A Calzado, Ed
British Journal of Pharmacology.2018; 175(19): 3813. CrossRef - EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis
Adela García-Martín, Martín Garrido-Rodríguez, Carmen Navarrete, Carmen del Río, María L. Bellido, Giovanni Appendino, Marco A. Calzado, Eduardo Muñoz
Biochemical Pharmacology.2018; 157: 304. CrossRef - Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
Kyoung Min Kim, Hyun-Jin Jin, Seo Yeon Lee, Hyo Jin Maeng, Gha Young Lee, Tae Jung Oh, Sung Hee Choi, Hak Chul Jang, Soo Lim
Endocrinology and Metabolism.2017; 32(3): 389. CrossRef - Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
Soo Lim, Kyoung Min Kim, Sin Gon Kim, Doo Man Kim, Jeong-Taek Woo, Choon Hee Chung, Kyung Soo Ko, Jeong Hyun Park, Yongsoo Park, Sang Jin Kim, Hak Chul Jang, Dong Seop Choi
Diabetes & Metabolism Journal.2017; 41(5): 377. CrossRef
- The modulation effects of plant‐derived bioactive ingredients on chronic kidney disease: Focus on the gut–kidney axis

- Obesity and Metabolism
- Transcriptional Regulation of Fibroblast Growth Factor 21 Expression
- Kwi-Hyun Bae, Jung-Guk Kim, Keun-Gyu Park
- Endocrinol Metab. 2014;29(2):105-111. Published online June 26, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.2.105
- 4,448 View
- 60 Download
- 25 Web of Science
- 24 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Fibroblast growth factor 21 (FGF21) is an attractive target for treating metabolic disease due to its wide-ranging beneficial effects on glucose and lipid metabolism. Circulating FGF21 levels are increased in insulin-resistant states; however, endogenous FGF21 fails to improve glucose and lipid metabolism in obesity, suggesting that metabolic syndrome is an FGF21-resistant state. Therefore, transcription factors for FGF21 are potential drug targets that could increase FGF21 expression in obesity and reduce FGF21 resistance. Despite many studies on the metabolic effects of FGF21, the transcriptional regulation of FGF21 gene expression remains controversial and is not fully understood. As the FGF21 transcription factor pathway is one of the most promising targets for the treatment of metabolic syndrome, further investigation of FGF21 transcriptional regulation is required.
-
Citations
Citations to this article as recorded by- Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production
Yu-Ming Chao, Hon-Yen Wu, Sin-Huei Yeh, Ding-I Yang, Lu-Shiun Her, Yuh-Lin Wu
International Journal of Molecular Sciences.2024; 25(8): 4211. CrossRef - Relationship between FGF21 and drug or nondrug therapy of type 2 diabetes mellitus
Chang Guo, Li Zhao, Yanyan Li, Xia Deng, Guoyue Yuan
Journal of Cellular Physiology.2021; 236(1): 55. CrossRef - Serum fibroblast growth factor 21 levels after out of hospital cardiac arrest are associated with neurological outcome
Pirkka T. Pekkarinen, Markus B. Skrifvars, Ville Lievonen, Pekka Jakkula, Laura Albrecht, Pekka Loisa, Marjaana Tiainen, Ville Pettilä, Matti Reinikainen, Johanna Hästbacka
Scientific Reports.2021;[Epub] CrossRef - Epigenetic Regulation of Processes Related to High Level of Fibroblast Growth Factor 21 in Obese Subjects
Teresa Płatek, Anna Polus, Joanna Góralska, Urszula Raźny, Agnieszka Dziewońska, Agnieszka Micek, Aldona Dembińska-Kieć, Bogdan Solnica, Małgorzata Malczewska-Malec
Genes.2021; 12(2): 307. CrossRef - Nutritional Regulation of Hepatic FGF21 by Dietary Restriction of Methionine
Han Fang, Kirsten P. Stone, Laura A. Forney, Desiree Wanders, Thomas W. Gettys
Frontiers in Endocrinology.2021;[Epub] CrossRef - The Presence of Urinary Ketones according to Metabolic Status and Obesity
Bo-Reum Kim, Jeong Woo Seo, Sang Man Kim, Kyu-Nam Kim, Nam-Seok Joo
Journal of Korean Medical Science.2020;[Epub] CrossRef - MS-275 induces hepatic FGF21 expression via H3K18ac-mediated CREBH signal
Qi Zhang, Qin Zhu, Ruyuan Deng, Feiye Zhou, Linlin Zhang, Shushu Wang, Kecheng Zhu, Xiao Wang, Libin Zhou, Qing Su
Journal of Molecular Endocrinology.2019; 62(4): 187. CrossRef - Spontaneous ketonuria and risk of incident diabetes: a 12 year prospective study
Gyuri Kim, Sang-Guk Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Ele Ferrannini, Yong-ho Lee, Nam H. Cho
Diabetologia.2019; 62(5): 779. CrossRef - Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges
Úrsula Martínez-Garza, Daniel Torres-Oteros, Alex Yarritu-Gallego, Pedro F. Marrero, Diego Haro, Joana Relat
International Journal of Molecular Sciences.2019; 20(19): 4692. CrossRef - Berberine-induced activation of AMPK increases hepatic FGF21 expression via NUR77
Feiye Zhou, Mengyao Bai, Yuqing Zhang, Qin Zhu, Linlin Zhang, Qi Zhang, Shushu Wang, Kecheng Zhu, Yun Liu, Xiao Wang, Libin Zhou
Biochemical and Biophysical Research Communications.2018; 495(2): 1936. CrossRef - Practical prospects for boosting hepatic production of the “pro-longevity” hormone FGF21
Mark F. McCarty
Hormone Molecular Biology and Clinical Investigation.2017;[Epub] CrossRef - The regulation of FGF21 gene expression by metabolic factors and nutrients
Anjeza Erickson, Régis Moreau
Hormone Molecular Biology and Clinical Investigation.2017;[Epub] CrossRef - Diabetes Mellitus and Sepsis
Silvia C. Trevelin, Daniela Carlos, Matteo Beretta, João S. da Silva, Fernando Q. Cunha
Shock.2017; 47(3): 276. CrossRef - The U-shaped relationship between fibroblast growth factor 21 and microvascular complication in type 2 diabetes mellitus
Chan-Hee Jung, Sang-Hee Jung, Bo-Yeon Kim, Chul-Hee Kim, Sung-Koo Kang, Ji-Oh Mok
Journal of Diabetes and its Complications.2017; 31(1): 134. CrossRef - Fibroblast Growth Factor 21—Metabolic Role in Mice and Men
Harald Staiger, Michaela Keuper, Lucia Berti, Martin Hrabě de Angelis, Hans-Ulrich Häring
Endocrine Reviews.2017; 38(5): 468. CrossRef - Anti-inflammatory effects of exercise training in adipose tissue do not require FGF21
Jay W Porter, Joe L Rowles, Justin A Fletcher, Terese M Zidon, Nathan C Winn, Leighton T McCabe, Young-Min Park, James W Perfield, John P Thyfault, R Scott Rector, Jaume Padilla, Victoria J Vieira-Potter
Journal of Endocrinology.2017; 235(2): 97. CrossRef - Hepatic Fgf21 Expression Is Repressed after Simvastatin Treatment in Mice
Panos Ziros, Zoi Zagoriti, George Lagoumintzis, Venetsana Kyriazopoulou, Ralitsa P. Iskrenova, Evagelia I. Habeos, Gerasimos P. Sykiotis, Dionysios V. Chartoumpekis, Ioannis G Habeos, Kostas Pantopoulos
PLOS ONE.2016; 11(9): e0162024. CrossRef - Association between insulin resistance and impairment of FGF21 signal transduction in skeletal muscles
Ja Young Jeon, Sung-E Choi, Eun Suk Ha, Tae Ho Kim, Jong Gab Jung, Seung Jin Han, Hae Jin Kim, Dae Jung Kim, Yup Kang, Kwan-Woo Lee
Endocrine.2016; 53(1): 97. CrossRef - Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury
Yanlong Liu, Cuiqing Zhao, Jian Xiao, Liming Liu, Min Zhang, Cuiling Wang, Guicheng Wu, Ming-Hua Zheng, Lan-Man Xu, Yong-Ping Chen, Moosa Mohammadi, Shao-Yu Chen, Matthew Cave, Craig McClain, Xiaokun Li, Wenke Feng
Scientific Reports.2016;[Epub] CrossRef - The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis
Kyung Mook Choi
Endocrinology and Metabolism.2016; 31(1): 1. CrossRef - Physiological and Pharmacological Roles of FGF21 in Cardiovascular Diseases
Peng Cheng, Fangfang Zhang, Lechu Yu, Xiufei Lin, Luqing He, Xiaokun Li, Xuemian Lu, Xiaoqing Yan, Yi Tan, Chi Zhang
Journal of Diabetes Research.2016; 2016: 1. CrossRef - Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases
Fangfang Zhang, Lechu Yu, Xiufei Lin, Peng Cheng, Luqing He, Xiaokun Li, Xuemian Lu, Yi Tan, Hong Yang, Lu Cai, Chi Zhang
Molecular Endocrinology.2015; 29(10): 1400. CrossRef - AMP-activated protein kinase suppresses the expression of LXR/SREBP-1 signaling-induced ANGPTL8 in HepG2 cells
Jinmi Lee, Seok-Woo Hong, Se Eun Park, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
Molecular and Cellular Endocrinology.2015; 414: 148. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production


 KES
KES

 First
First Prev
Prev



